Abstract
Introduction: Allogeneic stem cell transplantation (alloSCT) was the only curative option for younger patients with LBCL (DLBCL, tFL, PMBCL) relapsing or being refractory to second-line therapy including high-dose chemotherapy and autologous SCT. After two commercially available CART products became available many pts are receiving CART third line. We sought to compare data from the EBMT registry focussing on the relative role of both treatment modalities in > third-line treatment of LBCL.
Methods: Patients registered with the EBMT database from month 1/2016 to 5/2021 having received either a first alloSCT or commercially available CART therapy (Yescarta® or Kymriah ®) as ≥ third-line therapy of LBCL were analysed. To correct for imbalances in patient characteristics we did multivariate analyses considering only patient with complete information on IPI including LDH. Overall results in these patients and the population at large are identical.
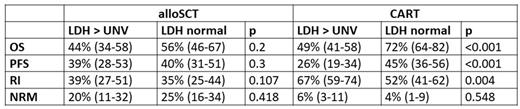
Results: We identified 1591 pts fulfilling the inclusion criteria (658 alloSCT, 933 CART); in 453 pts information on IPI and LDH at cell therapy was available (172 alloSCT and 281 CART). Patient groups differed significantly in clinical parameters as median age (50 yrs for alloSCT vs 62 yrs CART), median IPI score at cell therapy (1 in alloSCT and 3 in CART) and elevated LDH at cell therapy. The 1-year estimates for the major endpoints overall survival (OS), progression free survival (PFS), relapse incidence (RI) and non-relapse mortality (NRM) by the strongest prognostic factor LDH are given in the table below.
In multivariate analysis, the type of cellular therapy (CART vs alloSCT) (HR 0.71 (95% CI.53-0.96, p=0.0237)) and LDH normal vs elevated (HR 0.58 (95%CI 0.44-077, p=0.00018)) significantly influenced OS, whilst age was not significant. Regarding RI, CART do significantly worse than allo SCT (HR 1.74 (95% 1.26-2.38, P=0.00067)); NRM was significantly lower after CART (HR 0.23 (95%CI 0,12-0.45, p< 0.0001)).
Conclusion: Correcting for important confounding factors, pts given CART for ≥ 3rd line treatment of DLBCL still have better OS and PFS than pts treated with alloSCT. While CART therapy causes significantly less TRM, RI is generally higher than after alloSCT. Pts with active lymphoma (LDH >N, high IPI) prior to CART show high RI and a significant decrease in OS. This effect is less strong in allografted pts., Therefore, alloSCT remains a valuable option especially in patients with high-risk disease characterized by elevated LDH and high IPI.
Disclosures
Glass:Roche: Research Funding; Riemser: Research Funding; Roche: Honoraria; BMS: Honoraria; Gilead: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Miltenyi: Honoraria; JAZZ: Honoraria. Sureda:NOVARTIS: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; TAKEDA: Consultancy, Honoraria, Speakers Bureau; SANOFI: Consultancy, Honoraria; BMS: Consultancy, Honoraria; ROCHE: Consultancy, Honoraria; MSD: Honoraria; GILEAD: Consultancy. Dreger:Novartis: Honoraria; Kite: Honoraria. Corradini:abbvie: Honoraria; amgen: Honoraria; celgene: Honoraria; gilead: Honoraria; incyte: Honoraria; janssen: Honoraria; takeda: Honoraria. Ram:Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau. Kroeger:Novartis: Honoraria; Kite/Gilead: Honoraria. Wulf:Gilead: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Martín García-Sancho:Roche: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Gilead/Kite: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Servier: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Takeda: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Incyte: Consultancy, Other: Support for attending meetings and/or travel; Lilly: Consultancy, Other: Support for attending meetings and/or travel; ADC Therapeutics America: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Kyowa Kirin: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Miltenyi: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Kern: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Novartis: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Eusa Pharma: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Clinigen: Consultancy, Honoraria, Other: Support for attending meetings and/or travel. Stelljes:Medac: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Amgen: Consultancy; BMS: Consultancy, Honoraria; Kite: Consultancy, Honoraria. Forcade:Sanofi: Other: Travel Support; GSK: Speakers Bureau; MSD: Other: Travel Support; Novartis: Speakers Bureau; Jazz: Other: Travel Support, Speakers Bureau; Gilead: Other: Travel Support, Speakers Bureau. Finke:Riemser Pharma: Research Funding. Hilgendorf:Novartis: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Perez-Simon:PFIZER: Research Funding; ABBVIE: Research Funding; ALEXION: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; JAZZ: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; GILEAD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses. Schmitz:Janssen: Other: Research Support; Esteve: Honoraria; BMS: Other: Stocks publicly traded.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal